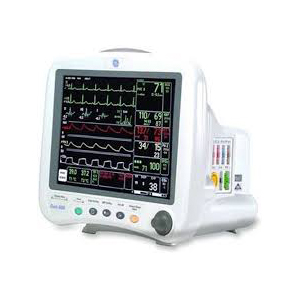
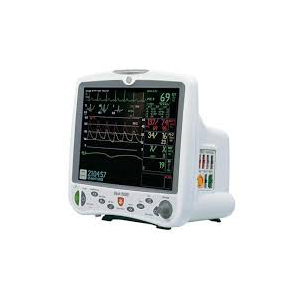
DIDCA Assembly - Edwards VIG/VIG2/VGLO Autoport
| 420915-052 | |
| New | |
| Patient Monitoring | |
| GE Healthcare | |
| GE Medical Systems Information Technologies | |
| GE HealthCare | |
| N/A | Outright |
Enter your approval number and submit to add item(s) to cart.
Please enter approval number
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without the
approval number, GE will contact you before your order
can be confirmed for shipment.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Features
- Does not contain Cadmium (Cd) more than 0.01% by weight, Lead (Pb), Mercury (Hg), Poly Brominated Biphenyl (PBB), Poly Brominated Diphenyl Ethers (PBDE), Bis(2-ethylhexyl) phthalate (DEHP), Butyl Benzyl Phthalate (BBP), Di Butyl Phthalate (DBP) and Di Iso Butyl Phthalate (DIBP) more than 0.1% by weight
- Material flammability rating of the circuit board follows UL 94V-0 standards and is classified as an essential for safety
- Safe packaging without any moisture in an anti-static bag
- Durable and reliable