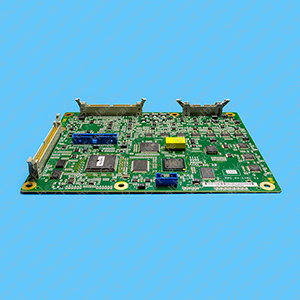
Jedi KV Control Spare5.47-4.26-P5.59A-RoHS 5436156
| 5436156 | |
| 2265276-5 2265276-6 5126994-3 5126994-4 5436156-H | |
| New | |
| X-Ray | |
| GE Healthcare | |
| GE Healthcare | |
| GE HealthCare | |
| N/A | Exchange - Return Defective |
|
Core Exchange | |
Enter your approval number and submit to add item(s) to cart.
Please enter approval number
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without the
approval number, GE will contact you before your order
can be confirmed for shipment.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Features
- Programmed kV control Printed Circuit Board Assembly (PCBA) FRU for X-ray generation systems
- Control board compatible to Jedi Silhouette VR, Jedi 50 Rad, Pioneer, Jedi 60CT and Proteus XR/A generator
- CD-ROM embedded firmware with "Floppy Tiger/Emperor P4.26 Upgrade Proteus P5.59A Upgrade"
- Included with high quality self adhesive part identification thermal transfer labels