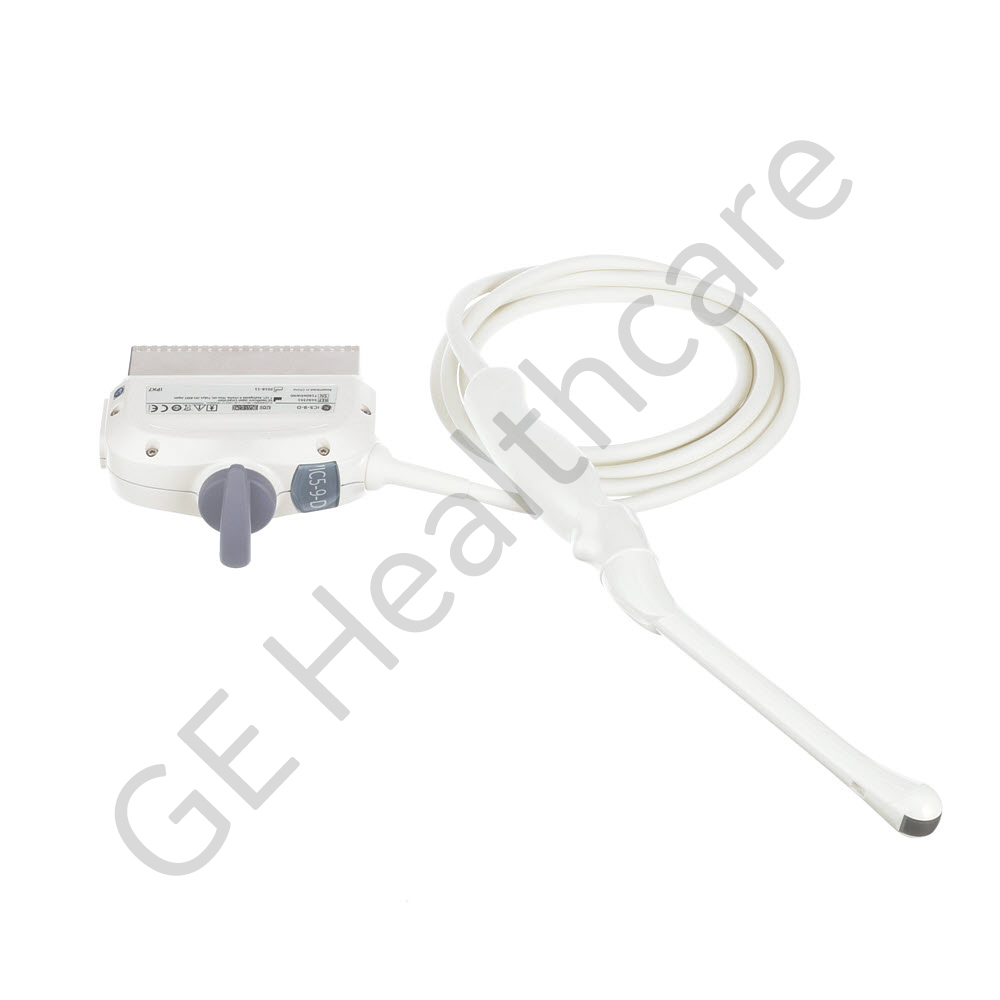
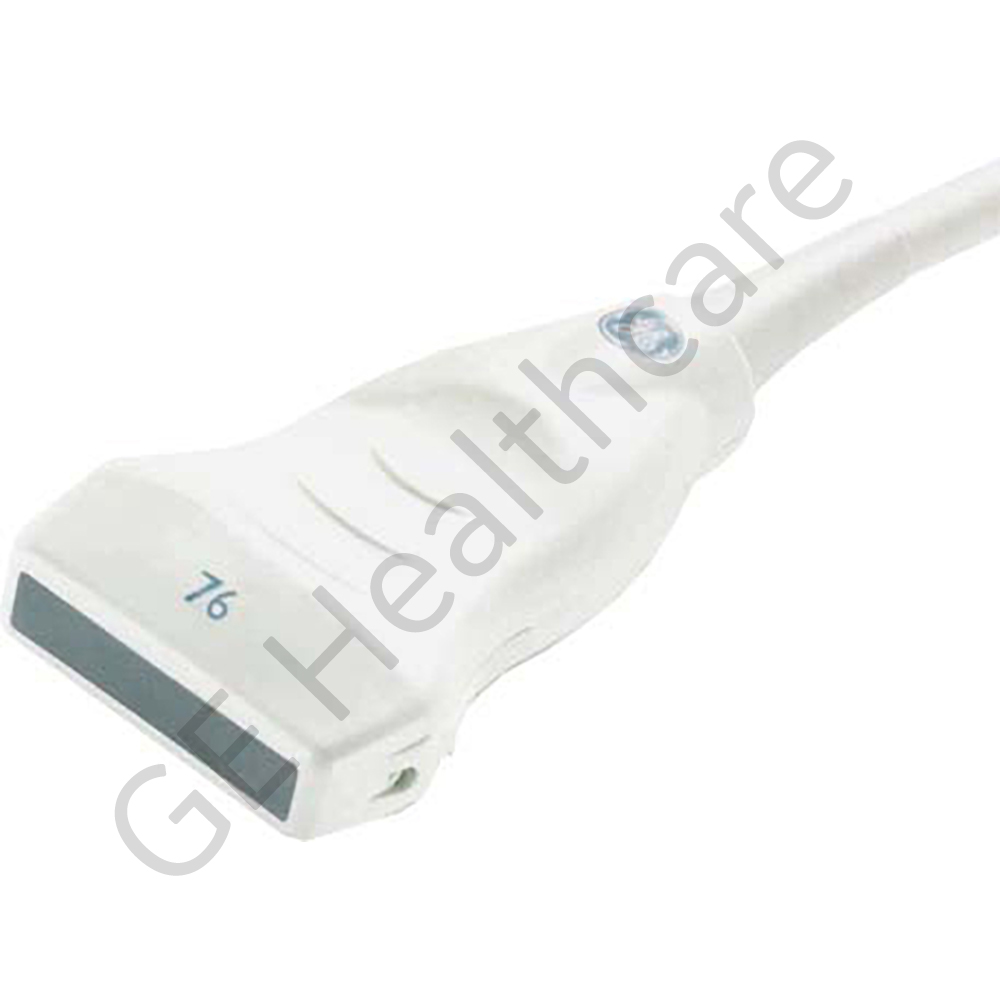
Castor Dual Lock 2 Polaris
| 5400392-2 | |
| 5400392 | |
| New | |
| Ultrasound | |
| LOGIQ S8 | |
| GE Healthcare | |
| GE Healthcare | |
| GE HealthCare | |
| N/A | Outright |
Enter your approval number and submit to add item(s) to cart.
Please enter approval number
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without the
approval number, GE will contact you before your order
can be confirmed for shipment.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Features
- Reliable and durable
- Free from internal sharp edges, scratches and notches
- Smooth wheel movement
- Strong, hard and can bear wide range of loads